Abstract

Introduction: Patients (pts) with transfusion-dependent β-thalassemia (TDT) have increased morbidity and mortality and reduced quality of life (QoL) compared with the general population owing to the mental, physical, and time demands of chronic transfusions and iron chelation. Autologous gene therapy with betibeglogene autotemcel (beti-cel) therapy uses a modified β-globin gene that establishes stable production of functional adult hemoglobin (Hb), thus allowing for transfusion independence (TI). Pts with TDT were treated with beti-cel in 2 completed phase 1/2 studies (HGB-204, HGB-205) and 2 ongoing phase 3 studies as of Aug 2021 (HGB-207, HGB-212). After 2 years of follow-up, pts could enroll in a long-term follow-up study (LTF-303 [NCT02633943]) for up to an additional 13 years. QoL improvement in the 1-2 years post beti-cel treatment was previously described. Here, we evaluate long term (3 years post-treatment) patient reported outcomes after beti-cel treatment in pts with TDT enrolled in LTF-303.
Methods: Pts with TDT underwent hematopoietic stem progenitor cell collection followed by myeloablative busulfan conditioning and beti-cel infusion. TI is defined as weighted average Hb ≥9 g/dL without packed red blood cell transfusions for ≥12 months. Generic health related QoL (HRQoL) over time was assessed using the Pediatric Quality of Life Inventory (PedsQL; range, 0-100) for pediatric and adolescent pts (<18 years old [YO]). Short Form-36 Health Survey Physical Component Summary and Mental Component Summary (SF-36 PCS and MCS, respectively; range 0-100), and the EuroQol (EQ-5D-3L) visual analog scale (VAS; range 0-100) composite index score (range 0-1) for adult (≥18 YO) pts. HRQoL was assessed at baseline (BL), Months (M) 6, 12, 18, 24 (in the parent HGB studies), and M 36, 48, and 60 (in LTF-303) after beti-cel infusion. Pts were excluded from HRQoL analyses if BL or M36 HRQoL assessment was missing as of data cut (Aug 2021). Higher scores indicated improvement in HRQoL. A bespoke questionnaire assessing β-thalassemia and pt-centric aspects of daily living (i.e., ability to work or attend school, physical activity, and perceived benefit from treatment) was completed at M 36.
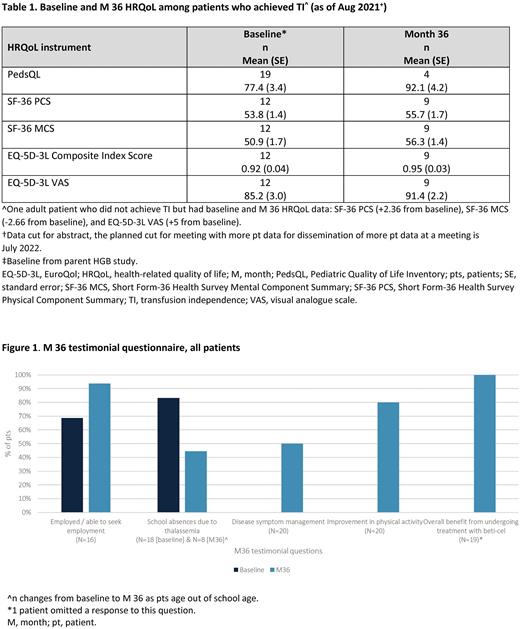
Results: As of August 18, 2021, 57 pts were enrolled in LTF-303 (phase 1/2: 22 pts; phase 3: 35 pts), of which 26 (45.6%) were pediatric / adolescent pts and 31 (54.4%) were adult pts. Among pts <18 YO with PedsQL data who achieved TI, the mean (standard error (SE)) PedsQL total score (population norm = 80.9) increased from 77.4 (3.4) at BL (n=19) to 92.1 (4.2) at M 36 (n=4). Among adult pts with SF-36 data who achieved TI, the mean (SE) SF-36 PCS and MCS scores (US general population norm = 50) increased from 53.8 (1.4) and 50.9 (1.7), respectively, at BL (n=12) to 55.7 (1.7) and 56.3 (1.4) at M 36 (n=9), respectively. The mean (SE) EQ-5D-3L Composite Index and VAS scores increased from 0.92 (0.04) and 85.2 (3.0), respectively, at BL (n=12), to 0.95 (0.03) and 91.4 (2.2) at M 36 (n=9) (Table 1). Data from the M 36 testimonial questionnaire (Figure 1) showed the ability to seek employment or be employed, increased from 68.8% (11/16) of pts at BL to 93.8% (15/16) at M 36. There was a reduction in school absences at M 36 (44.4% [4/9] missing school) compared with BL (83.3% [15/18] missing school). 50% (10/20) of pts still required management of disease symptoms at M 36. 80% (16/20) of pts reported improvement in physical activity at M 36. 100% (19/19) of pts reported an overall benefit from undergoing treatment with beti-cel.
Conclusions: Published data shows beti-cel is potentially curative in pts with TDT, defined as having TI with normal or near-normal Hb. It was previously shown that with high-range QoL at BL, QoL improved at 1-2 years post-beti-cel. The updated data indicates durability of QoL improvement through year 3. A trend towards a positive impact on employment, school attendance, physical activity, and other aspects of life is also observed.
Disclosures
Locatelli:Novartis: Honoraria, Speakers Bureau; Jazz Pharmaceuticals: Honoraria; Medac: Speakers Bureau; SOBI: Speakers Bureau; Miltenyi: Speakers Bureau; BlueBird bio: Speakers Bureau; Amgen: Speakers Bureau; Neovii: Speakers Bureau. Walters:BioLabs, Inc.: Consultancy; Vertex Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Ensoma, Inc.: Consultancy, Current holder of stock options in a privately-held company; AllCells, Inc.: Consultancy. Kwiatkowski:Agios: Consultancy; bluebird bio, Inc.: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy; Celgene: Consultancy; Imara: Consultancy, Research Funding; Forma: Consultancy, Research Funding; Chiesi: Consultancy; Silence Therapeutics: Consultancy; Apopharma: Research Funding; Bioerativ: Research Funding; Sangamo: Research Funding; Biomarin: Consultancy; CRISPR/Vertex: Research Funding. Cavazzana:Smart-Immune: Current Employment, Current equity holder in private company; Cellectis: Consultancy; Noga: Consultancy. Porter:Protagonism: Honoraria; Celgene: Consultancy, Honoraria; bluebird bio: Consultancy, Honoraria; VIFOR: Honoraria; Silence Therapeutics: Honoraria; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; La Jolla Pharmaceuticals: Honoraria; Agios: Consultancy, Honoraria; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees. Thrasher:Orchard Therapeutics: Consultancy; Rocket Pharmaceuticals: Consultancy; 4bio capital: Consultancy; Generation Bio: Consultancy, Current equity holder in publicly-traded company. Kulozik:Vertex: Honoraria; BMS: Honoraria; bluebird bio, Inc.: Honoraria, Research Funding; Celgene: Honoraria. Thuret:bluebird bio, Inc.: Other: Participation to clinical trials; Celgene: Other: Participation to clinical trials; Novartis pharma: Other: Participation to clinical trials; Vertex Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees. Rasko:Genea: Current equity holder in publicly-traded company; Rarecyte: Current equity holder in private company; Gilead, Roche, Novartis, Bluebird Bio, SPARK therapeutics, Cynata, Pfizer Inc: Consultancy. Yannaki:Pfizer: Speakers Bureau. Ali:bluebird bio, Inc.: Current Employment. Thakar:bluebird bio, Inc.: Current Employment. Gruppioni:bluebird bio, Inc.: Current Employment. Thompson:Global Blood Therapeutic: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Editas: Research Funding; Biomarin: Research Funding; Baxalta: Research Funding; CRISPR/Vertex: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; bluebird bio, Inc.: Consultancy, Research Funding; Beam: Consultancy; Agios: Consultancy; Novartis: Research Funding.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal